This site is not intended for US customers. US customers,
click here.Not all products and indications may be licensed in your country. For more information contact your local representative.
In the EU, the following products are registered under different names: EMSCULPT (BTL EMSCULPT), EXION (BTL-785F), EMFACE (BTL-785F), EMSELLA (BTL EMSELLA).
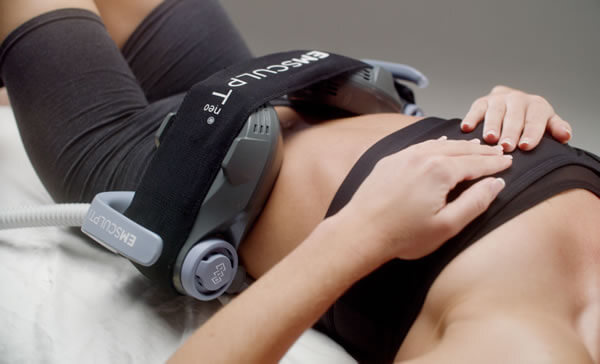
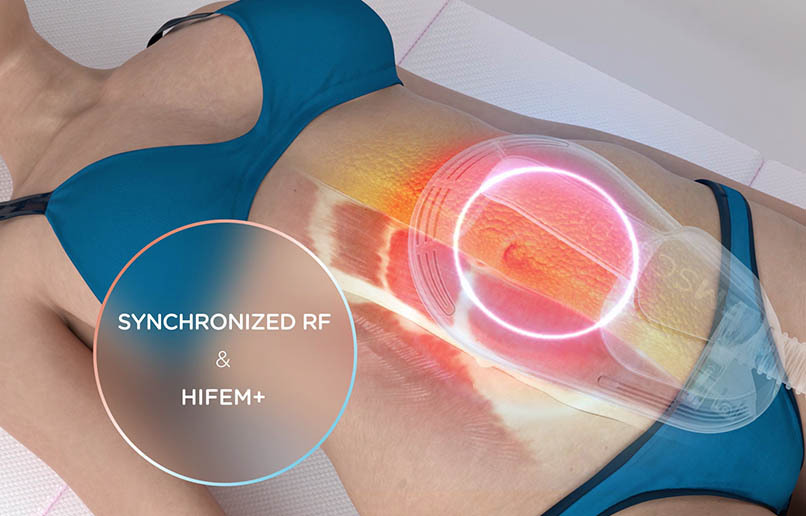
BTL®, EXILITE®, EXILIS®, EXION™, VANQUISH®, UNISON®, CELLUTONE®, CORE TO FLOOR®, EMSCULPT®, EMSCULPT NEO®, EMSELLA®, EMFACE®, EMFEMME 360®, EMBODY®, EMTONE® and HIFEM® are registered trademarks in the United States of America, the European Union and/or other countries. The products, the methods of manufacture or the use may be subject to one or more U.S. or foreign patents or pending applications, see www.btlnet.com/patents. Trademarks EMSCULPT®, EMSCULPT NEO®, EMSELLA®, EMFACE®, EMTONE®, EMBODY®, EMFEMME 360™, and HIFEM® are parts of EM™ Family of products.*Data on file. **The procedure runs independently once applicators are affixed. Clients must not be left unattended during an active treatment.
Results and patient experience may vary. As with any medical procedure, ask your doctor if the procedure is right for you.
© 2024, BTL Group of Companies. All rights reserved |
Terms of Use